Safety Profile
Serious and common adverse reactions (ARs)
Serious ARs associated with PIQRAY include severe hypersensitivity, severe cutaneous adverse reactions (SCARs), hyperglycemia, pneumonitis, diarrhea or colitis, and embryo-fetal toxicity.1
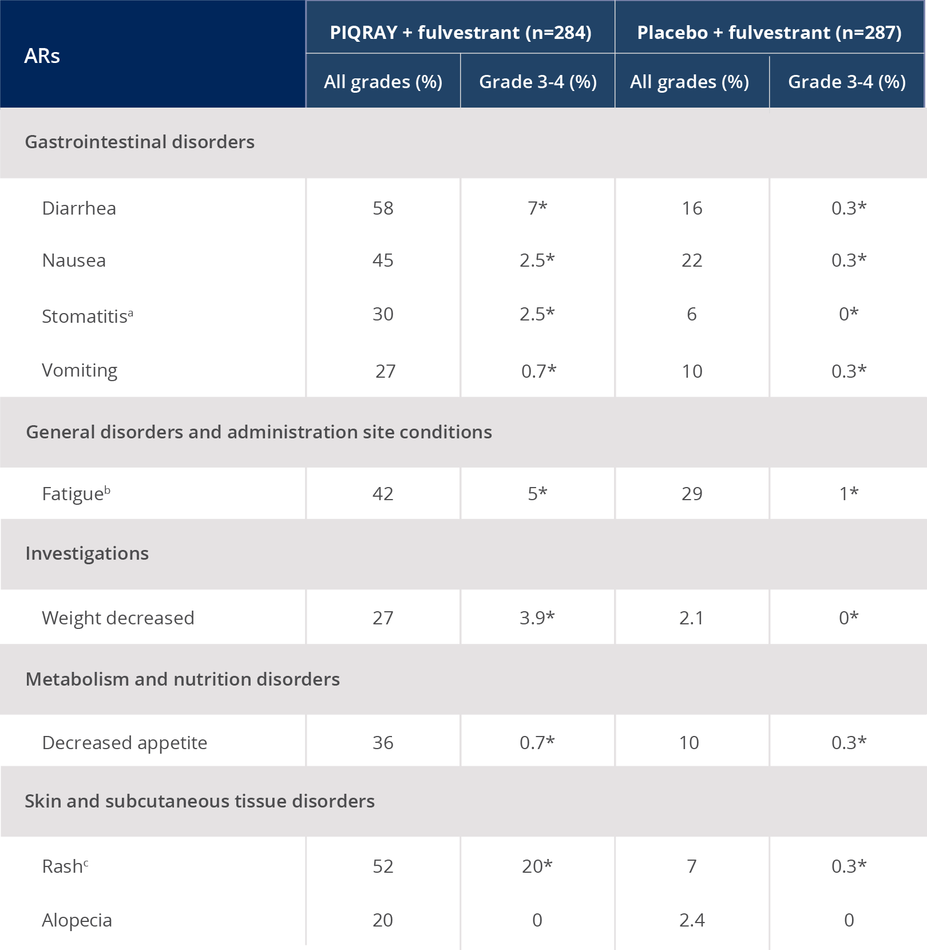
ARs occurring in >20% of the total population1
*No grade 4 ARs were reported.
aIncluding stomatitis, aphthous ulcer, mouth ulceration.
bIncluding fatigue, asthenia.
cIncluding rash, rash maculo-papular, rash macular, rash generalized, rash papular, rash pruritic.
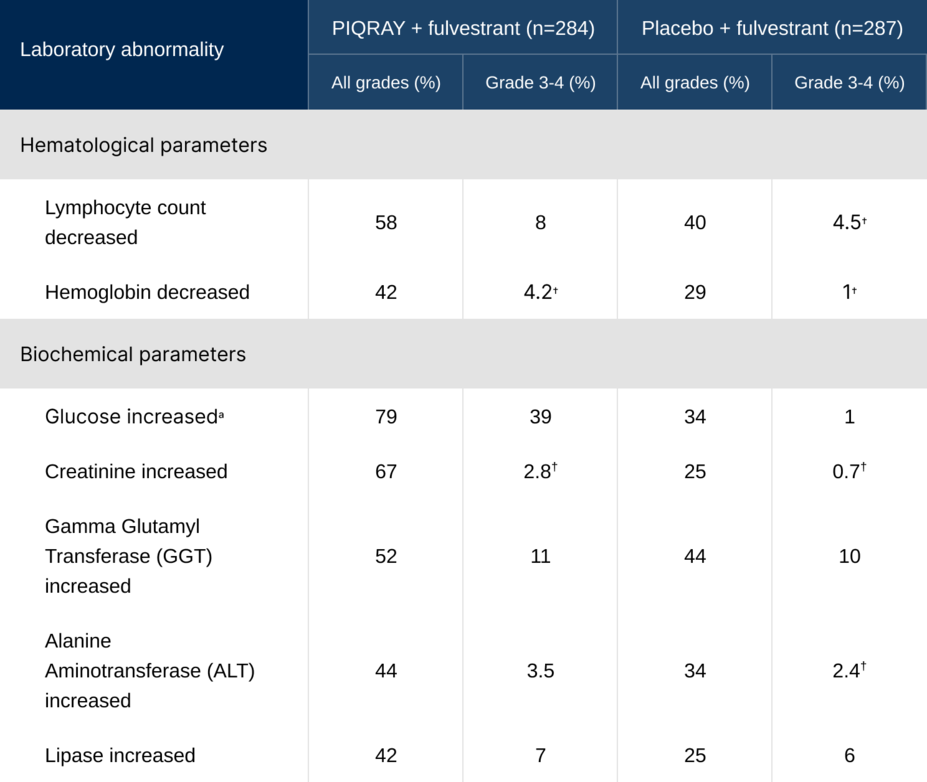
Laboratory abnormalities occurring in >30% of the total population1
†No grade 4 laboratory abnormalities were reported.
aGlucose increase is an expected laboratory abnormality of PI3K inhibition. Hyperglycemia, a laboratory-related AR, was reported in 65% of patients treated with PIQRAY (grade 3=33%; grade 4=3.9%).
Glucose increase, including hyperglycemia, is an expected, on-target effect of PI3K inhibition1,2
Prophylactic use of metformin 7 days prior to initiating PIQRAY was observed to decrease the incidence and severity of hyperglycemia1
- Ketoacidosis was reported in 0.7% of patients (n=2) treated with PIQRAY1
Among patients treated with PIQRAY and fulvestrant, 5% permanently discontinued both therapies and 21% permanently discontinued PIQRAY alone due to ARs1
- The most common ARs leading to discontinuation of PIQRAY were hyperglycemia (6% of patients), rash (4%), diarrhea (3%), and fatigue (3%)1
Dose reductions due to ARs occurred in 55% of patients receiving PIQRAY and fulvestrant1
- The most common ARs leading to a dose reduction of PIQRAY were hyperglycemia (29% of patients), rash (9%), diarrhea (6%), stomatitis (4%), and mucosal inflammation (2%)
Patient management resources

BROCHURE
Monitoring AR Checklist
Monitor for ARs before and during treatment

BROCHURE
Managing Patients on PIQRAY
Take an in-depth look at managing selected ARs